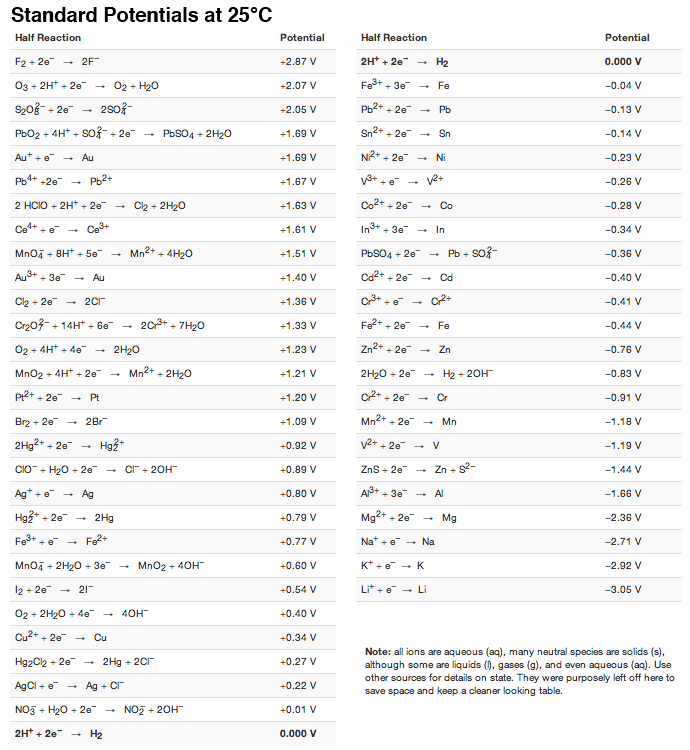
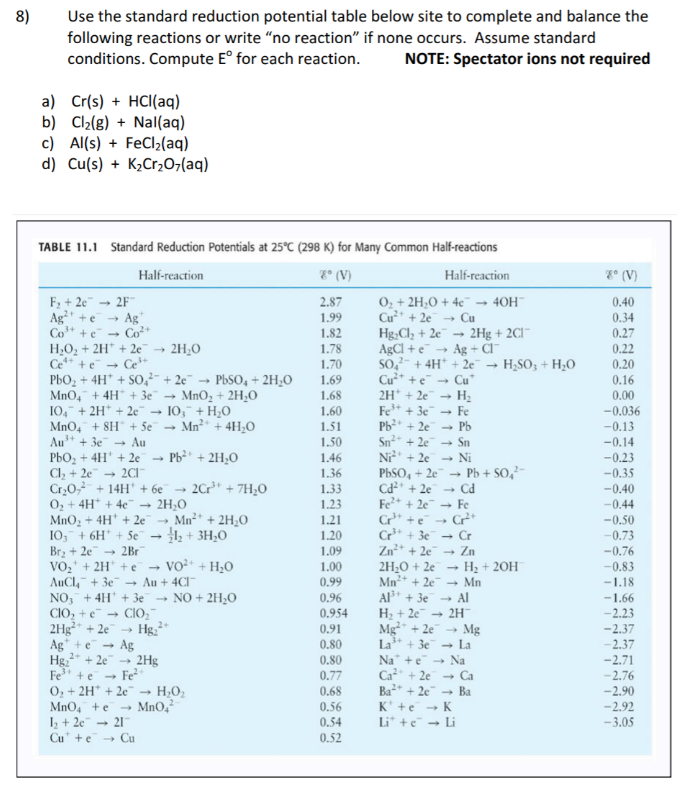
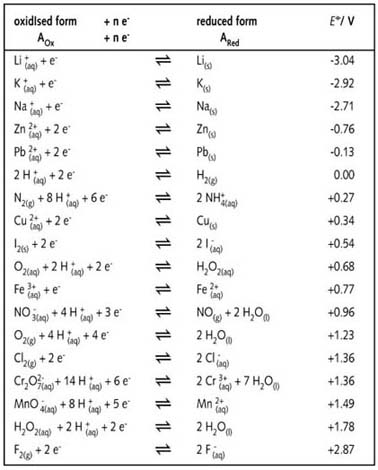
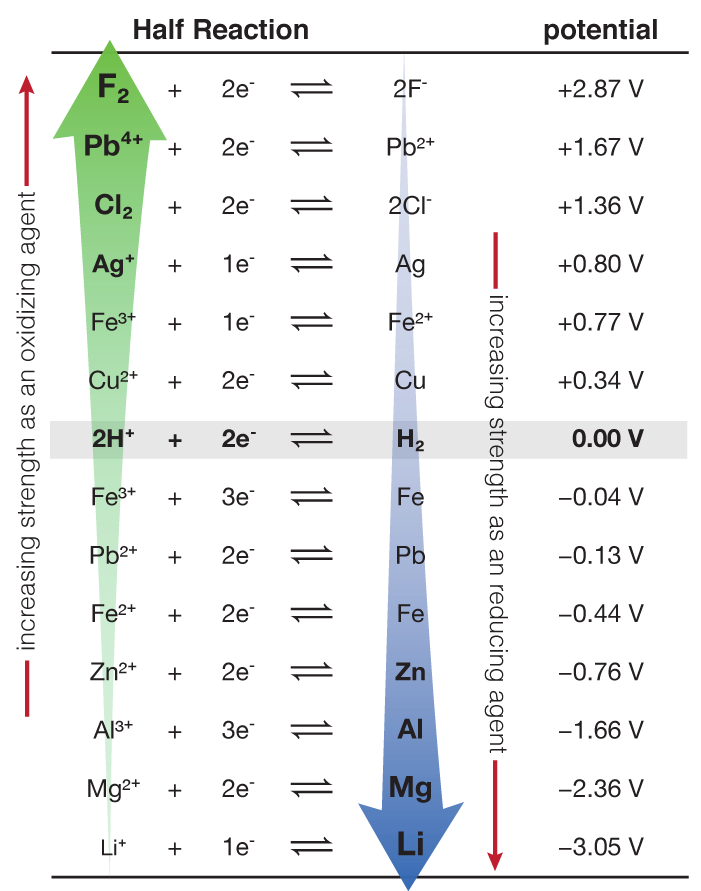
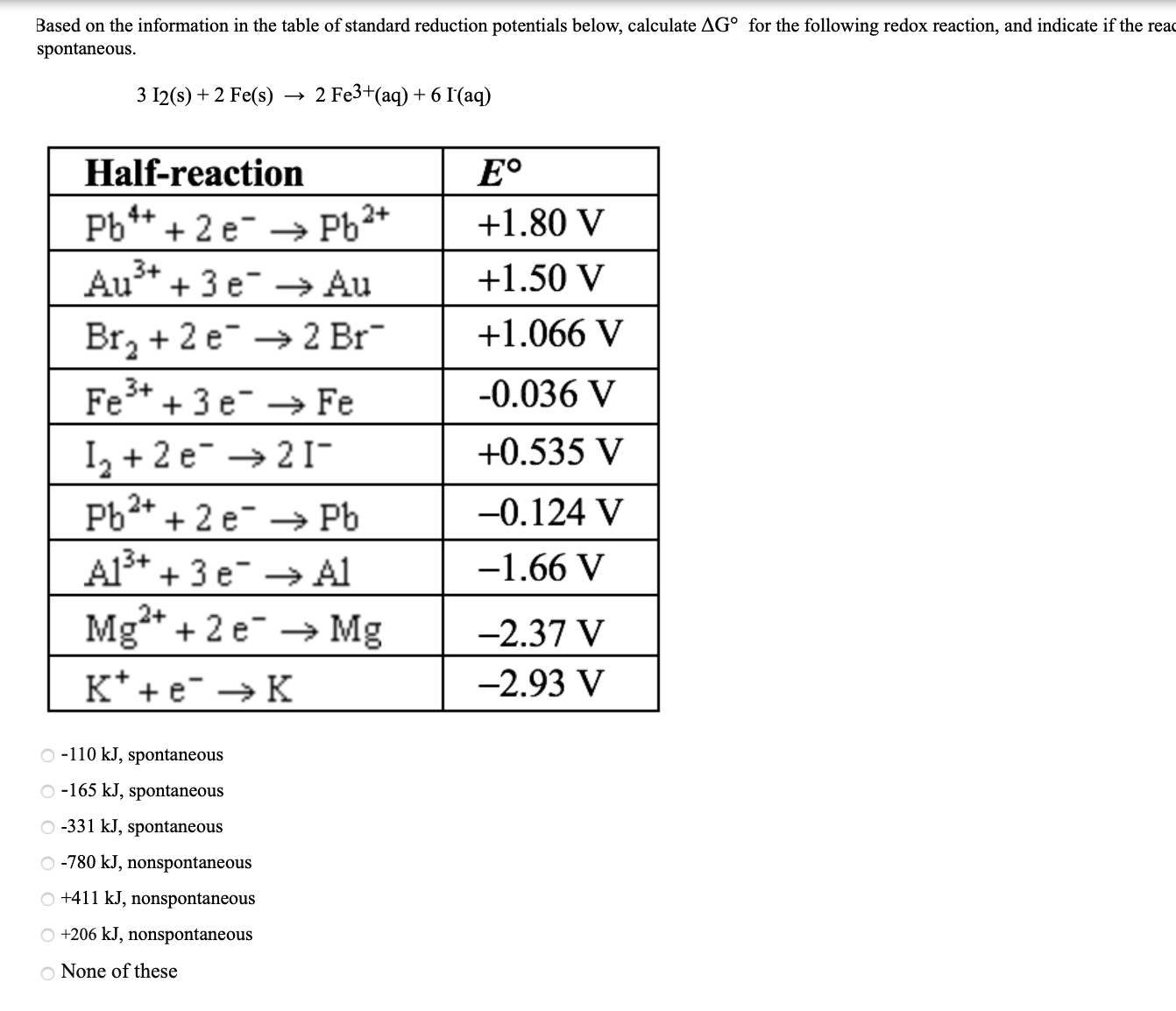
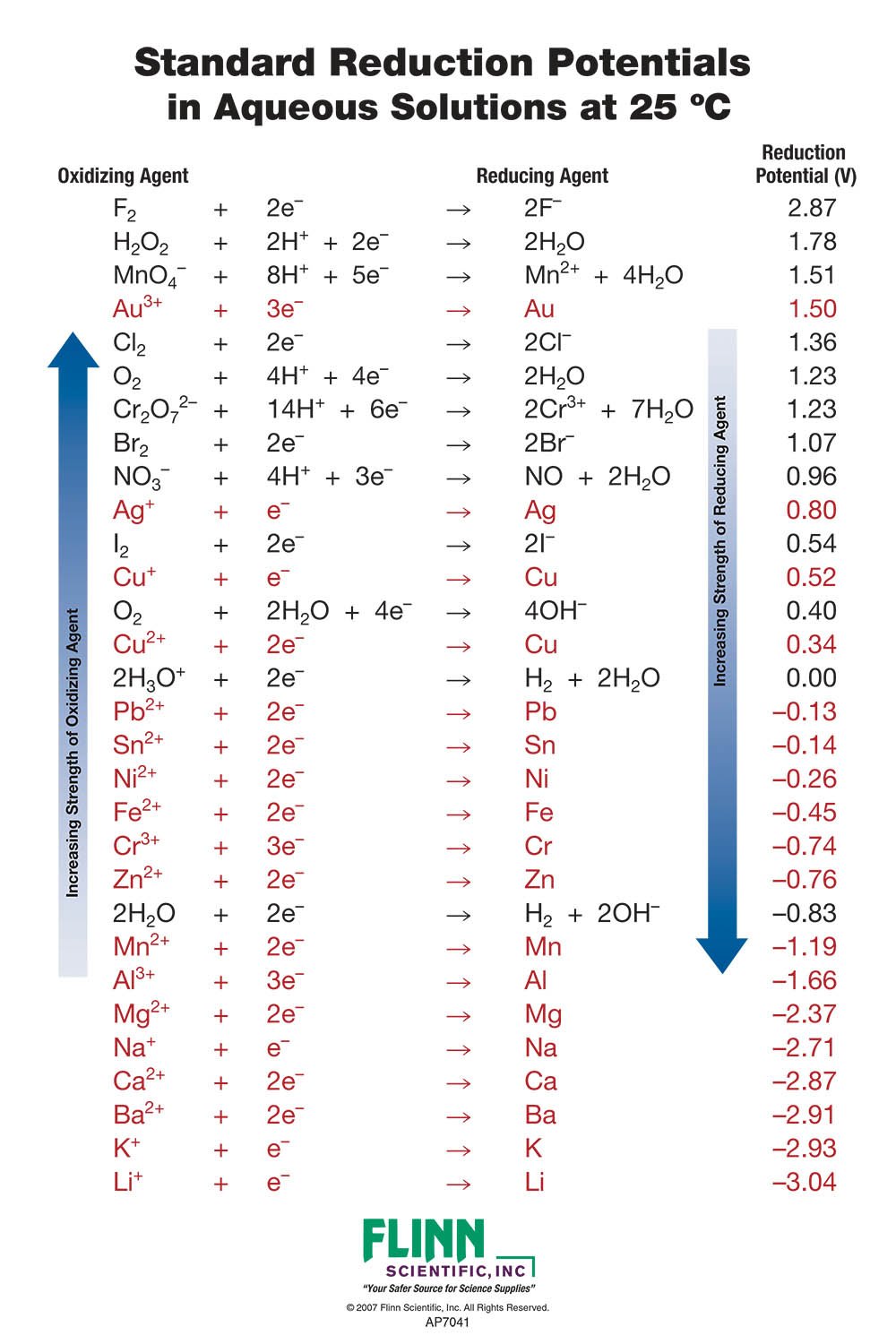
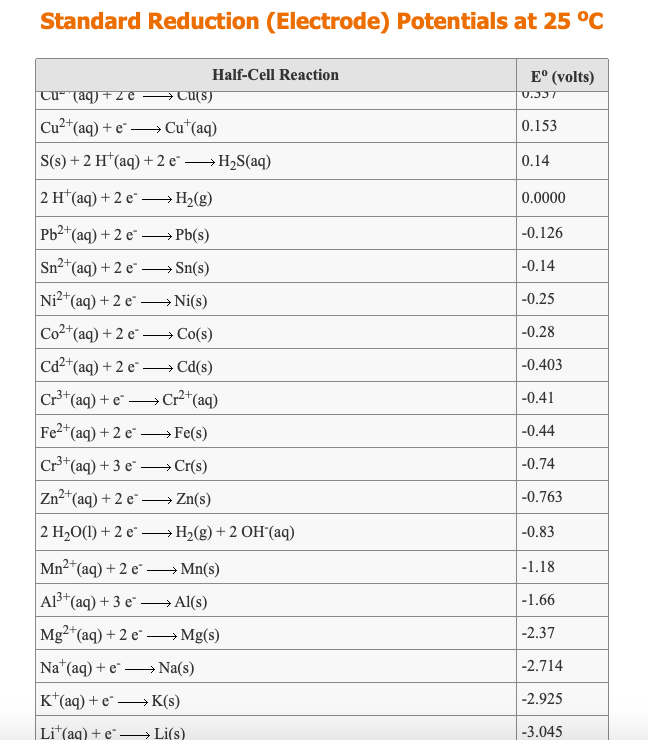
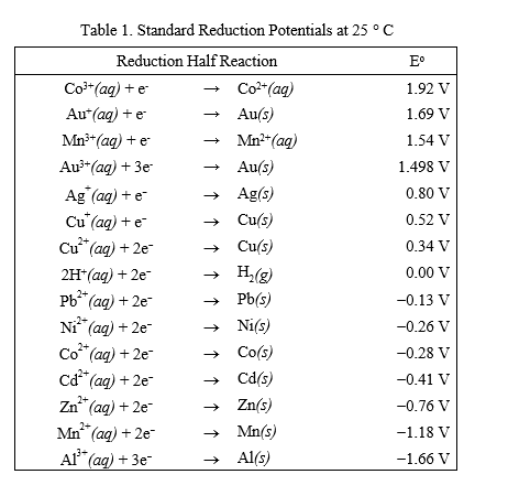
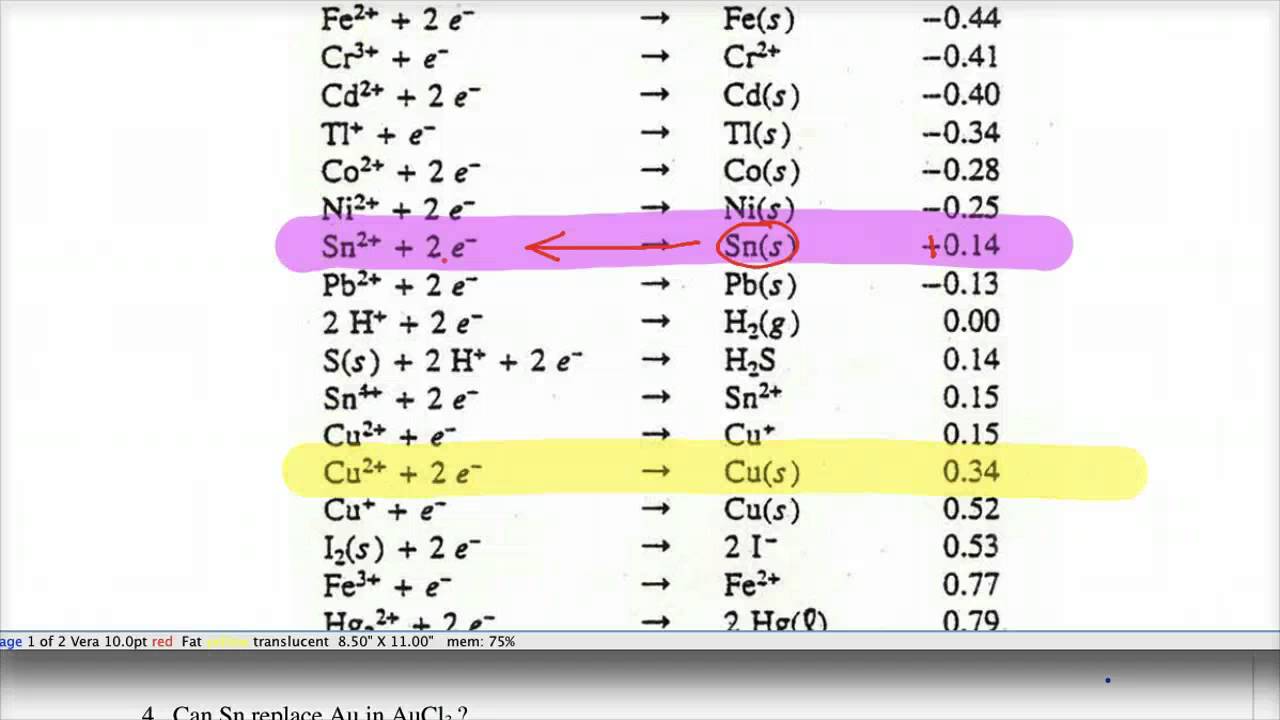
Using the standard electrode potentials given in the table, predict the reaction between the following is possible.Fe^{3+}(aq) and I^{-}(aq)

Table 1 from Absolute standard hydrogen electrode potential measured by reduction of aqueous nanodrops in the gas phase. | Semantic Scholar

SOLVED: Table A5.5 Standard Reduction Potentials at 25°C (298 K) for Many Common Half-Reactions Half-Reaction Half-Reaction 4OH- + F2 â†' 2F- + 2H2O 2.87 2H2O + 4e- â†' Cu2+ + 2OH- 0.01
Using the standard electrode potentials given in the Table, predict the reaction between the following is feasible.Ag(s) and Fe^{3+}(aq)

Table 4 from Estimation of standard reduction potentials of halogen atoms and alkyl halides. | Semantic Scholar
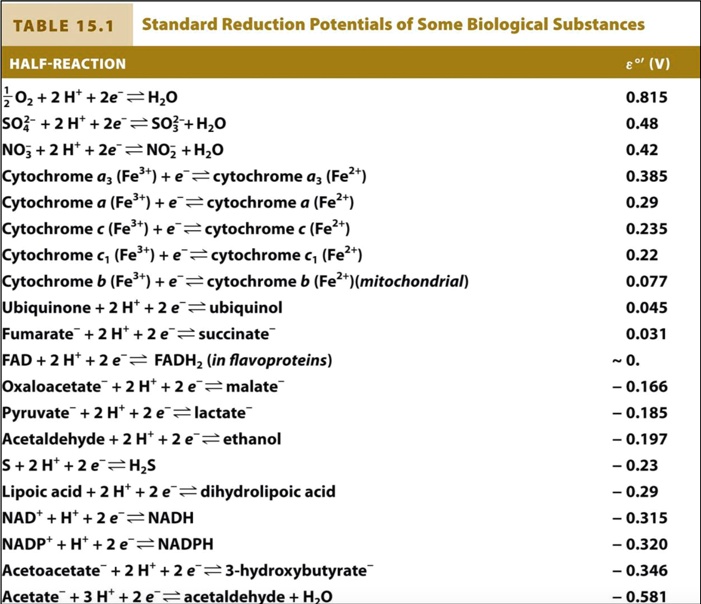
SOLVED: TABLE 15.1 Standard Reduction Potentials of Some Biological Substances HALF-REACTION (V) O2 + 2H+ + 2e- = H2O SO4^2- + 2 H+ + 2e- = H2SO3 NO3- + 2 H+ +