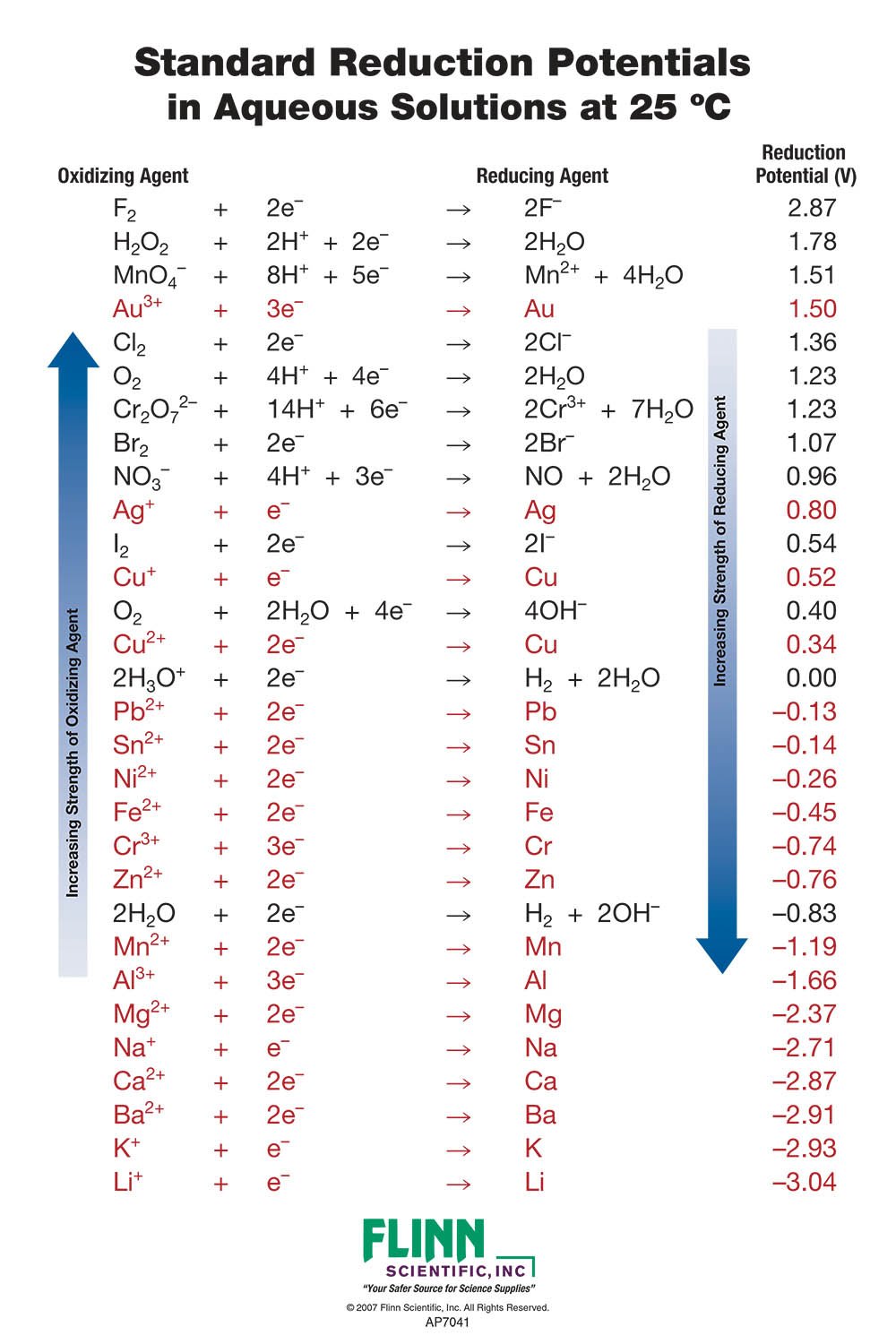
Table 6-3 from Redox potential and mobility of contaminant oxyanions (As, Sb, Cr) in argillaceous rock subjected to oxic and anoxic cycles | Semantic Scholar

Table 1 from Tungsten's redox potential is more temperature sensitive than that of molybdenum. | Semantic Scholar

Table 1 from Redox potentials of primary electron acceptor quinone molecule (QA)− and conserved energetics of photosystem II in cyanobacteria with chlorophyll a and chlorophyll d | Semantic Scholar


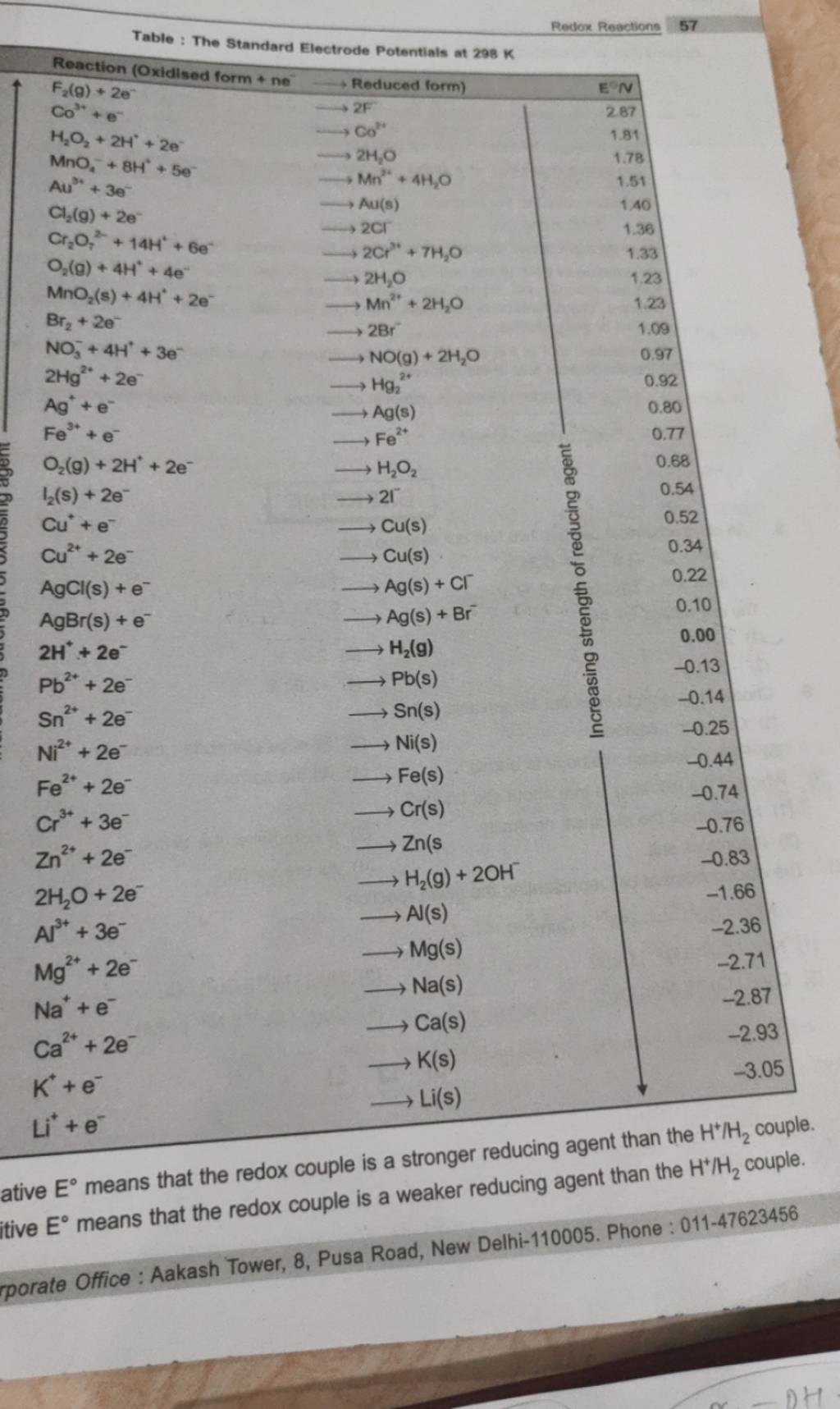



![AUFBAU1 [REFERENCE SECTION: REDOX POTENTIALS] AUFBAU1 [REFERENCE SECTION: REDOX POTENTIALS]](https://www.wissensdrang.com/media/tablerp.gif)